Surface expression of the HLA-I related molecules MICA and MICB (MICA/B) in response to oncogenic and cellular stress acts as a natural anti-cancer immunosurveillance mechanism. The recognition of MICA/B by the activating immunoreceptor NKG2D, which is expressed by natural killer (NK) and T cell subsets, is responsible for the removal of many transformed and virally infected cells. However, tumors frequently evade NKG2D-mediated immunosurveillance by proteolytic shedding of MICA/B, which can inhibit NKG2D function and promote tumor immune escape. Recently, we demonstrated that monoclonal antibodies targeting the conserved, membrane-proximal α3 domain of MICA/B can prevent MICA/B shedding and enhance NK cell anti-tumor efficacy. With the goal of leveraging the ubiquity of MICA/B expression on malignant cells, we have developed a novel chimeric antigen receptor targeting the α3 domain of MICA/B (CAR-MICA/B) and are currently evaluating application of CAR-MICA/B in an off-the-shelf NK cell immunotherapy platform for both solid and hematopoietic tumor indications.
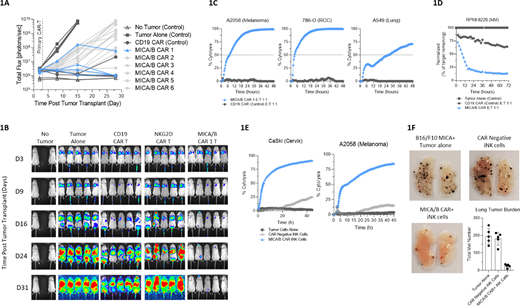
Optimization of CAR-MICA/B design was performed by primary T cell transduction using a matrix of CAR spacers and ScFv heavy and light chain orientations. Six candidate CAR-MICA/B designs were screened in vitro against a panel of tumor cell lines and in vivo against the Nalm6 leukemia cell line engineered to express MICA (Nalm6-MICA). All tested constructs demonstrated MICA-specific in vitro activation and cytotoxicity as well as in vivo tumor control (Figure 1A). Additional studies utilizing the optimal CAR-MICA/B configuration demonstrated MICA/B-specific reactivity against a panel of solid and hematopoietic tumor cell lines in vitro, including melanoma, renal cell carcinoma, and lung cancer lines (Figure 1B). Further, CAR-MICA/B T cells were superior to NKG2D-CAR T cells in clearing A2058 melanoma cells in an in vivo xenograft metastasis model (Figure 1C).
Although MICA/B expression has primarily been studied in the context of solid tumors, moderate MICA/B mRNA expression was identified in a number of hematopoietic tumor cell lines, including acute myeloid leukemia (AML) and multiple myeloma (MM) lines. Following the confirmation of surface MICA/B protein expression on a selection of MM and AML cell lines, we utilized MICA/B CAR primary T cells to further demonstrate MICA/B-specific activation and cytotoxicity and to confirm CAR-MICA/B targeting of hematological malignancies (Figure 1D).
To further advance CAR-MICA/B development, we introduced the CAR-MICA/B construct into an induced pluripotent stem cell (iPSC) line designed for production of off-the-shelf natural killer (NK) cell immunotherapies. Using a panel of tumor cell lines expressing MICA/B, CAR-MICA/B iPSC-derived NK (iNK) cells displayed specific MICA reactivity, resulting in enhanced cytokine production, degranulation, and CAR-mediated cytotoxicity compared to CAR-negative iNK control cells (Figure 1E). In addition to MICA/B-specific cytotoxicity mediated by CAR, iNK cells also mediated innate cytotoxicity against cancer cells through endogenous NKG2D and other NK cell activating receptors, highlighting the multifaceted targeting capacity of CAR iNK cells. In order to isolate CAR-directed cytotoxicity from the iNK cells' innate anti-tumor capacity, an in vivo proof of concept study was performed using mouse B16-F10 melanoma cells engineered to express human MICA. In this model, iNK expressing CAR-MICA/B significantly reduced B16-F10-MICA liver and lung metastases from CAR-MICA/B iNK cells compared to CAR negative control cells, with reductions of the number of metastases by 87% in the lung (p<0.0001) and 93% in the liver (p<0.006) for CAR-MICA/B iNK cells vs non-CAR controls (Figure 1F). Additionally, CAR-MICA/B iNK cells were effective at controlling Nalm6-MICA progression in a disseminated leukemia model, suggesting potential application against both hematopoietic and solid tumors. Ongoing work is focused on extending these studies into disease-specific models of endogenous MICA/B expression to further advance CAR-MICA/B iNK cells in both solid and hematologic cancers. In summary, these preclinical data support the development and translation of an off-the-shelf NK cell immunotherapy targeting the conserved α3 domain of MICA/B with potential therapeutic application to multiple hematopoietic and solid tumor types.
Bjordahl:Fate Therapeutics: Current Employment. Goulding:Fate Therapeutics: Current Employment. Blum:Fate Therapeutics: Current Employment. Chang:Fate Therapeutics: Current Employment. Wucherpfennig:Fate Therapeutics: Research Funding. Chu:Fate Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company; Roche Holding AG: Current equity holder in publicly-traded company. Chu:Fate Therapeutics, Inc: Current Employment. Gaidarova:Fate Therapeutics, Inc: Current Employment. Liu:Fate Therapeutics: Current Employment. Sikaroodi:Fate Therapeutics: Current Employment. Fong:Fate Therapeutics: Current Employment. Huffman:Fate Therapeutics: Current Employment. Lee:Fate Therapeutics, Inc.: Current Employment. Valamehr:Fate Therapeutics, Inc: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal